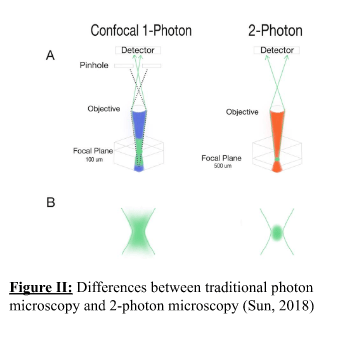
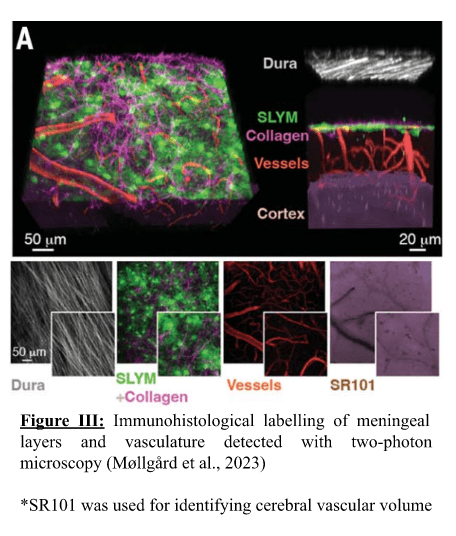
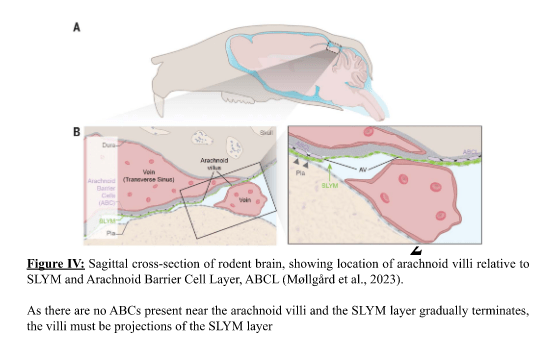
Zahra Mohsin, Second Year Medicine
Elizabeth Blackwell made history as the first woman in America to receive a medical degree, (Michals, 2015), as well as the first to have their name entered in the British General Medical Council’s Register in 1859, (University of Bristol, n.d.). Yet despite being a pioneer for women in the medical field, many may be unaware of the contributions which Dr Elizabeth Blackwell made towards promoting rights of women and their education in the medical profession.
Blackwell was born in Bristol, England on February 3, 1821, the third of nine children to Samuel Blackwell, wealthy owner of a sugar refinery, and his wife Hannah Lane, (Michals, 2015). Although unusual for the era, her father insisted that Blackwell and her siblings be equally well educated, (BBC, 2008), resulting in her receiving an excellent education provided by private tutors, (WAMS, n.d.). In 1832, the family emigrated from Bristol to New York after the failure of her father’s business, moving again a few years later to Cincinnati, Ohio, (Michals, 2015). Blackwell’s father died in 1838, leaving his family in financial hardship and, following his death, she and her sisters took to teaching and opened a private school to support their family, (University of Bristol, n.d.).
During her mid-20s, a close companion passed away from a prolonged illness, and prior to her passing, she had confided in Blackwell that her suffering would have been lessened had she been treated by a female doctor, (WAMS, n.d.). Following this, Blackwell decided to devote her career to studying medicine and ensuring that women received high quality healthcare. She began studying medicine privately for a few years before seeking admission to medical school, (Thakur et al., 2024). After several rejections, she was admitted to Geneva Medical College after the faculty, assuming they would not allow for a female to be enrolled, permitted the all-male student body to vote on her admission. As a joke, the student body voted “yes,” and Blackwell subsequently became a medical student, (University of Bristol, n.d.).
Blackwell faced discrimination and hostility throughout her time at medical school, including being forced to sit separately during lectures and often being excluded from labs, (Michals, 2015). Despite these odds, she continued to persevere, ultimately ranking first in her class in 1849, (The Editors of Encyclopedia Britannica, n.d.).
In the same year, Blackwell travelled to Paris where she studied midwifery at La Maternité. Here she contracted a serious eye infection whilst attending to a newborn, resulting in her becoming blind in one eye and ultimately compelling her to abandon hopes of becoming a surgeon, (Thakur et al., 2024). She later returned to England and worked under Dr, (later Sir), James Paget at St Bartholomew’s Hospital, (The Editors of Encyclopedia Britannica, n.d.). She became increasingly interested in social causes, particularly regarding the education of women, (Luft, n.d.). In the summer of 1851, she went back to the United States where prejudice against female physicians made practising medicine difficult, as she was refused posts and was unable to rent private consulting quarters, (The Editors of Encyclopedia Britannica, n.d.). Despite it taking a long time to develop her private practice, Blackwell opened a small dispensary in a slum district in New York in 1853, later being joined by her younger sister, Dr Emily Blackwell, and by Dr Marie E. Zakrzewska (The Editors of Encyclopedia Britannica, n.d.). In 1857, the dispensary was incorporated as the New York Infirmary for Women and Children. This was a healthcare facility dedicated to providing accessible healthcare for underserved populations, whilst also serving as a professional environment for female physicians, medical students, and nursing scholars (Thakur et al., 2024).
During a year-long lecture tour of Great Britain, Blackwell became the first woman to have her name on the British Medical Register in 1859, (The Editors of Encyclopedia Britannica, n.d.), becoming a pioneer for British women wanting to join the medical profession. In 1861, she also helped organise the Women’s Central Association of Relief and the U.S. Sanitary Commission to help select and train nurses during the outbreak of the American Civil War, (The Editors of Encyclopedia Britannica, n.d.). As an advocate for gender equality in medical education, Blackwell argued that women should be allowed to study in the same recognised institutions as their male counterparts, (Thakur et al., 2024). Henceforth, The Woman’s Medical College of the New York Infirmary opened in 1868 with a total of fifteen students and nine teaching staff, including Blackwell as a professor of hygiene (Thakur et al., 2024). In 1869, Blackwell moved back to England, leaving the college to be run by her sister Emily, (Thakur et al., 2024).
Blackwell founded the National Health Society in 1871; this aimed to educate people on the benefits of hygiene and healthy lifestyles, something which she was passionate about (University of Bristol, n.d.). Their motto, “prevention is better than cure” is one which still holds value today, and highlights the longevity of Blackwell’s legacy.
In 1874, alongside British physicians Sophia Jex-Blake and Elizabeth Garret Anderson, Blackwell established the London School of Medicine for Women (University of Bristol, n.d.), being appointed as a professor of gynaecology. Over the next years of her life, she also spent time writing and publishing books and pamphlets on subjects including hygiene, family planning, preventative medicine, sanitation, and medication education (University of Bristol, n.d.). She died on the 31st of May 1910 in Hastings, England.
Elizabeth Blackwell spent her life dedicated to a profession which many deemed unsuitable and unattainable. Nevertheless, she spent her life advocating not only for her own prospects, but for the rights of others, with a passion and enthusiasm that helped shape a place for women in medicine. Her legacy and commitment is one that is, and should be continued to be, recognised within the medical field and beyond.
References
- BBC 2008. BBC – Bristol – History – The Bristol girl who became an American legend. bbc.co.uk. [Online]. [Accessed 9 January 2025]. Available from: https://www.bbc.co.uk/bristol/content/articles/2006/09/20/blackwell_feature.shtml.
- Library of Congress 1877. Elizabeth Blackwell, 1821-1910, oval bust, wearing wedding veil [Online]. [Accessed 9 January 2025]. Available from: https://www.loc.gov/item/2005679734/.
- Luft, E. v.d n.d. Celebrating 150 Years of Women in Medicine | Elizabeth Blackwell | HWS. http://www.hws.edu. [Online]. [Accessed 9 January 2025]. Available from: https://www.hws.edu/about/history/elizabeth-blackwell/150-years.aspx.
- Michals, D. 2015. Elizabeth Blackwell. National Women’s History Museum. [Online]. [Accessed 9 January 2025]. Available from: https://www.womenshistory.org/education-resources/biographies/elizabeth-blackwell.
- Thakur, J., Choudhari, S.G. and Gaidhane, A. 2024. Pioneering Physician: Dr. Elizabeth Blackwell and the Path to Medical History. Cureus. [Online]. 16(9). [Accessed 9 January 2025] Available from: https://doi.org/10.7759/cureus.68713.
- The Editors of Encyclopedia Britannica n.d. Elizabeth Blackwell | Biography & Facts. Encyclopædia Britannica. [Online]. [Accessed 9 January 2025]. Available from: https://www.britannica.com/biography/Elizabeth-Blackwell.
- University of Bristol n.d. Who was Elizabeth Blackwell? Bristol.ac.uk. [Online]. [Accessed 9 January 2025]. Available from: https://www.bristol.ac.uk/blackwell/who-we-are/who-was-elizabeth-blackwell/.
- WAMS n.d. Life Story: Elizabeth Blackwell. Women & the American Story. [Online]. [Accessed 9 January 2025]. Available from: https://wams.nyhistory.org/a-nation-divided/civil-war/elizabeth-blackwell/.